BARDA partners with Hoxworth Blood Center to develop and validate an improved method to evaluate next-generation blood products
To further prioritize the advanced development of next-generation blood products for use, BARDA is partnering with Hoxworth Blood Center, University of Cincinnati, to develop an improved alternative method to assess blood products in regulatory studies.
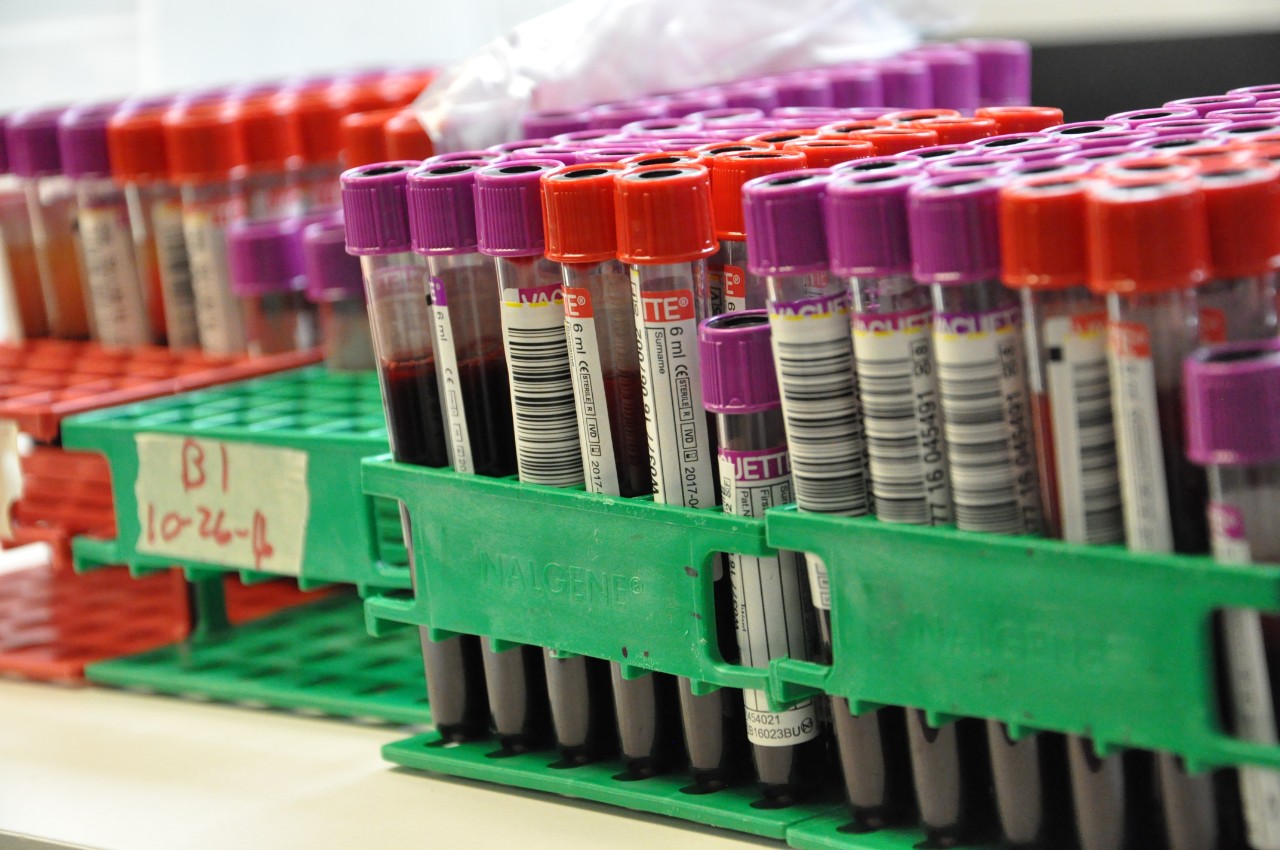
Hoxworth Blood Center will conduct pre-clinical and clinical trials to compare Biotinylation to Chromium-51 to evaluate the safety and efficacy of using a non-radioactive alternative labeling method to assess blood products. If biotin is found to be a more effective labeling alternative, it could result in many improvements of safety and availability of blood products in radiological or nuclear mass casualty incidents (MCI).
Biotin (vitamin B7) is an essential nutrient that helps the human body metabolize fatty acids, glucose, and amino acids and is naturally present in food and is available as a dietary supplement. Since it is more widely found, it could help improve the amount of blood products available for patients that need it. Additionally, the U.S. has only one producer of Chromium-51 which could result in less product availability and potential shortages.
Cr-51 is a synthetic radioactive isotope of chromium used to label RBCs to facilitate measurement of circulating RBC mass, volume, and survival time. The use of Cr-51 exposes research subjects and laboratory technicians to radiation and limits the number of laboratories permitted to perform testing due to regulation requirements, such as needing to obtain a special license and may be subject to state or U.S. Nuclear Regulatory Commission guidelines for possessing/using radioactive materials. Additionally, more laboratories could evaluate blood products and novel blood products candidates, including those for use in transfusion in the context of public health emergencies and routine medical treatment and would be a safer alternative to using Cr-51 because it is not radioactive.
Under the agreement, BARDA will provide funding and expertise for a clinical trial to validate biotin, a naturally occurring vitamin, as a safe and effective option to replace chromium 51 (Cr-51) as the U.S. Food and Drug Administration’s (FDA’s) current “gold standard” labeling method for measurement of circulating red blood cells (RBCs). BARDA invests in next generation blood product candidates that address hematopoietic injuries and build blood system capacity, including those that have long shelf-lives, are stable at higher temperatures, and can be administered in the field or enroute to a hospital. These investments rely on enabling technologies, such as safer labeling methods that enable evaluation of circulating RBCs, to support regulatory approval.
This award contributes to objectives set in BARDA’s 2022-2026 Strategic Plan, which emphasizes the importance of supporting the development of enabling technologies that can facilitate the regulatory process.
This award is one component of the BARDA’s CBRN Division medical countermeasure portfolio, visit the CBRN Portfolio to learn more.
About Hoxworth:
Hoxworth Blood Center, University of Cincinnati, was founded in 1938 and serves more than 30 hospitals in 18 counties in Southwestern Ohio, Northern Kentucky, and Southeastern Indiana. Annually, Hoxworth collects more than 100,000 units of blood from local donors to help save the lives of patients in area hospitals. Hoxworth Blood Center: Saving Lives Close to Home.
Related Stories
We love ‘Lucy’ — the AI avatar redefining UC tech transfer
July 17, 2024
In a visionary leap at the University of Cincinnati, the marriage of artificial intelligence and interactive technology has birthed "Lucy," a Smarthelp AI avatar poised to revolutionize how regional industries engage with UC's tech transfer initiatives.
NIS program opens new horizons for international student
July 17, 2024
In his pursuit of physics and a taste for research, Akash Khanikor ventured from his hometown in India's Assam to the University of Cincinnati, drawn by the promise of hands-on exploration early in his undergraduate career as a NEXT Innovation Scholar.
Camp aims to empower children, teens who stutter
July 17, 2024
A one-week, evidence-based program for children and teens who stutter at the University of Cincinnati will teach kids to communicate effectively, advocate for themselves and develop confidence about their communication abilities. Camp Dream. Speak. Live., which is coming to Cincinnati for the first time July 22-26, began in 2014 at the University of Texas at Austin. The Arthur M. Blank Center for Stuttering Education and Research at UT expects to serve more than 2,000 children at camps across the United States, Africa, Asia and Europe this year.
UC archivist explores Troy’s invisible workers
July 17, 2024
UC Classics archivist Jeff Kramer examined the unheralded and largely uncredited role laborers played in the 1930s excavation at Troy in Turkey.
CCM alum Donald Lawrence to be inducted into Cincinnati Black Music Walk of Fame
July 16, 2024
UC College-Conservatory of Music alumnus Donald Lawrence is part of the 2024 class of Cincinnati Black Music Walk of Fame inductees. Located at the Banks in downtown Cincinnati, the induction ceremony on July 27 will feature a parade of stars and a free concert by the Zapp Band.
U.S. stroke survival is improving, but race still plays role
July 16, 2024
U.S. News & World Report, HealthDay and Real Health covered new research from the University of Cincinnati that found overall rates of long-term survival following stroke are improving, but Black individuals experience worse long-term outcomes compared to white individuals.
Presidential challenge to UC: Join Ride Cincinnati to fight cancer
July 16, 2024
UC President Neville Pinto has again challenged every UC college and unit to send at least one rider to the September 14 Ride Cincinnati event to help fundraise for cancer research and cancer care. UC students ride free. Signup by July 31 for free UC-branded cycling jersey.
Pediatric ICU rates linked to housing quality, income, education
July 16, 2024
Healio highlighted research led by the University of Cincinnati and Cincinnati Children's Hospital's Carlie Myers that found a link between pediatric ICU admission rates and housing quality, household income and education.
UC study: Long-term stroke survival improving, but racial disparities remain
July 15, 2024
New research from the University of Cincinnati published in the journal Neurology found long-term survival rates following acute ischemic strokes are improving, but Black individuals experience worse long-term outcomes compared to white individuals.
Students organize to shake up Parkinson's care model
July 15, 2024
University of Cincinnati student and Parkinson's Together founder Mallika Desai joined the Parkinson's Experience Podcast to discuss the nonprofit's origins and multidisciplinary mission to meet the needs of patients in their community.
