UC research leader participates in COVID-19 vaccine trial
Dean for clinical research literally rolls up his sleeves to help overcome the pandemic
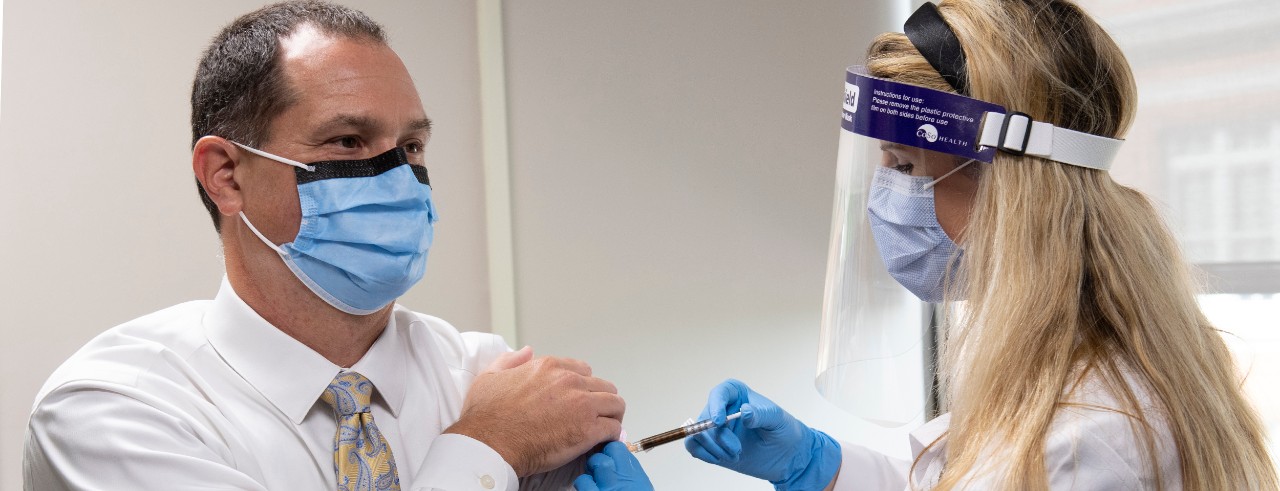
Brett Kissela, MD, pushes up the fabric on the arm of his dress shirt. He sits on an exam table on the fourth floor of Holmes Hospital, located on the University of Cincinnati’s medical campus.
“Is that enough?” he asks, as the pharmacist prepares the needle, which could contain either a vaccine for COVID-19 or a placebo. The first doses of the trial were administered last week.
“What made you interested in the trial?” asks Mary Burns, PharmD, pharmacist with UC Health, administering the dose.
“Science,” says Kissela, smiling under his mask.
The senior associate dean for clinical research at the UC College of Medicine and chief of research services at UC Health served as an example for the research he conducts, becoming a participant in the vaccine trial, sponsored by Moderna, Sept. 1. Moderna is the Massachusetts-based biotechnology company focused on drug discovery, development and vaccine technologies.
"The only way out of this is through science,” he says. “With over 20 years of experience as a researcher, this is a time when I can personally make a difference and help advance the field from the other side of the fence.”

Brett Kissela, MD, has his blood drawn as part of the clinical trial process. Photo/Colleen Kelley/UC Creative + Brand
With over 20 years of experience as a researcher, this is a time when I can personally make a difference and help advance the field from the other side of the fence.
Brett Kissela, MD

Brett Kissela, MD, participates in the COVID-19 vaccine trial at UC. He poses here with UC Health nurse Eva Whitehead. Colleen Kelley/UC Creative + Brand
Patients who participate in the blinded, randomized, placebo-controlled study will receive either the vaccine, or a placebo, in two injections. The vaccine is incorporated into the patient’s cells, which should begin producing a viral antigen. Then, the patient’s immune system can respond by making antibodies that will protect against future viral infection. Patients will keep a daily electronic diary of their symptoms, and also participate in telehealth and clinic visits with UC Health clinicians over a two-year period.
“We have to do this the right way, in order to make sure it works, and create an effective vaccine that will help our entire population,” he says. “I’m excited to be part of it.”
Featured photo of Brett Kissela, MD, by Colleen Kelley/UC Creative + Brand
Impact Lives Here
Those eligible for the COVID-19 vaccine trial include adults at least 18 years of age with no known history of SARS-CoV-2 infection, but are at risk of becoming infected. In addition to healthy individuals, patients at risk for severe COVID-19 disease, such as those with diabetes, heart or lung disease, or other chronic medical conditions, will also be included in the study.
For information or to enroll in the study, click here, call 513-245-3417, or email UCcovidresearch@uchealth.com.
Related Stories
Bearcats Racing speeds toward a victorious season
April 12, 2025
Bearcats Motorsports, Bearcats Baja and Bearcats EV are racing to build winning car models at UC’s 1819 Innovation Hub as competition season approaches.
Doctors prepare for surgeries with 3D-printed organs
April 11, 2025
Meteora3D, a Venture Lab-backed startup, helps surgeons better understand upcoming procedures by designing and developing quick-to-produce, 3D-printed anatomical models.
CEAS Expo unveils inaugural innovation awards – discover the...
April 11, 2025
During the CEAS Expo’s first-ever 1819 Innovation Awards, five groundbreaking inventions received recognition. Learn about prize winners from the CEAS Expo and their transformative projects.
